CDC ends universal newborn hepatitis B vaccination recommendation
Agency approves individual-based decision-making for birth-dose vaccine; antibody-testing recommendation remains under review
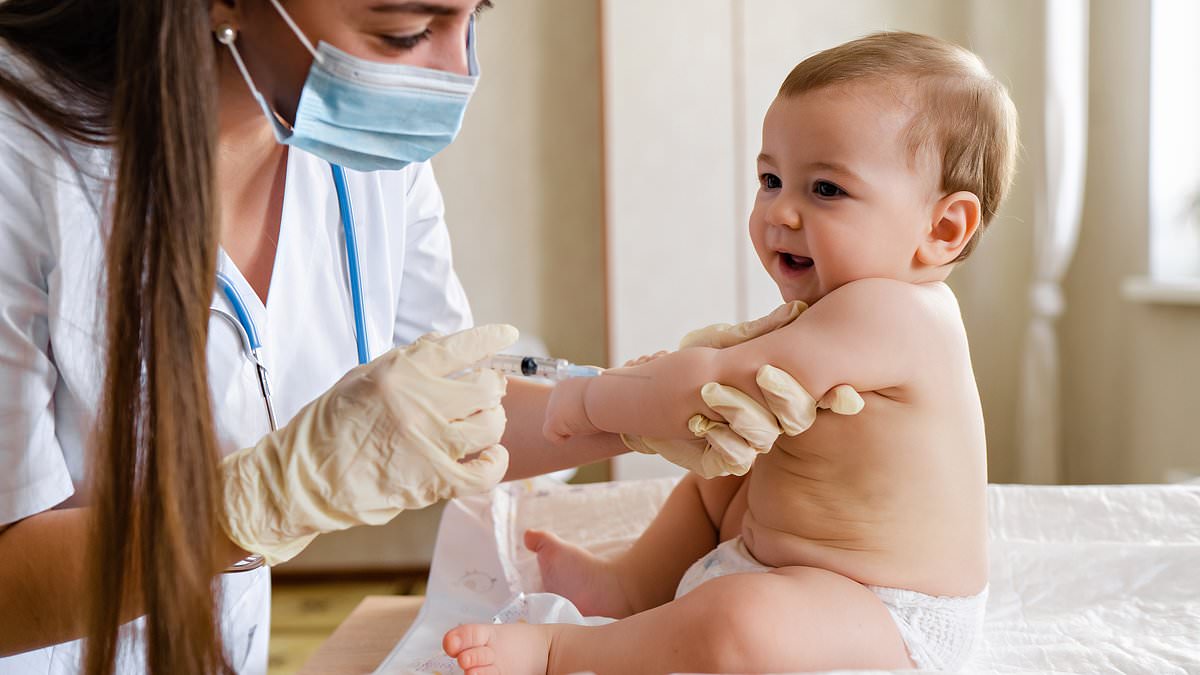
The Centers for Disease Control and Prevention formally dropped its blanket recommendation that all newborns receive the hepatitis B vaccine within 24 hours of birth. Acting CDC Director Jim O’Neill approved ending the universal birth-dose mandate on Tuesday, saying vaccination should be guided by individual decision-making for babies born to parents without hepatitis B. The birth dose remains an option for all infants but is now explicitly allowed to be delayed until at least two months of age in most cases. Children born to mothers with hepatitis B will still be advised to receive the vaccine at birth. The CDC did not endorse ACIP’s concurrent recommendation to test newborns for hepatitis B antibodies to determine whether additional doses are needed.
Hepatitis B is a viral infection that attacks the liver and can cause lifelong scarring, liver failure and cancer. It remains incurable, though vaccines prevent infection in most cases. Estimates suggest about 2.4 million adults in the United States are infected with hepatitis B, with about 640,000 living with chronic infection. In infants, up to 90 percent of those infected can develop chronic disease. The hepatitis B vaccine is given in three doses and has shown protection in more than 95 percent of healthy infants, children and young adults in clinical trials, according to health authorities.
The change follows a recommendation from ACIP, the federal advisory committee on immunization practices, made two weeks earlier. O’Neill said the revised policy “restores the balance of informed consent to parents whose newborns face little risk of contracting hepatitis B.” He added that the agency is still reviewing ACIP’s second recommendation regarding antibody testing to guide additional doses.
ACIP has come under intense scrutiny for months, in part because of its membership, which critics say was hand-picked by vaccine-skeptic Health and Human Services Secretary Robert F. Kennedy Jr. The panel’s stance on the birth dose has drawn outcry from medical associations that argue there is little evidence to support delaying the dose at birth.
Pediatricians have reported that more parents are declining the birth dose, the American Academy of Pediatrics has said. Susan Kressly, president of the American Academy of Pediatrics, said delaying the dose could undermine protection for infants who remain at risk in the first months of life and stressed the importance of timely vaccination for those at higher risk.
Major health insurers have indicated they will continue to cover all vaccines that were recommended at the start of 2025 through 2026, including the hepatitis B birth dose, regardless of the policy change.
The United States approved the hepatitis B vaccine in 1982 and has recommended the three-dose schedule for infants since 1991. In 2005, the policy was tightened to require the first dose within 24 hours of birth, regardless of the mother’s hepatitis B status. Previously, the first dose could be given at birth or between one and two to three months of age. At ACIP’s recent meeting, some members highlighted that hepatitis B is a serious disease with significant health consequences, while others cautioned about administering the birth dose to newborns born to mothers without the disease. They noted the United States’ diversity, absence of universal health care, and the country’s large population as factors complicating comparisons with other high-income nations, such as the United Kingdom and Denmark, where the first dose is often delayed to two months of age. Canada was discussed as a close peer that makes hepatitis B vaccine recommendations on a state-by-state basis. The agency said it is continuing to monitor the situation and will provide updates as more data become available.
About 91.4 percent of US children have received all three hepatitis B doses by age 24 months, according to vaccination data. Estimates indicate roughly 20,000 infants are born to hepatitis B–infected mothers each year, with higher proportions among foreign-born or non-white populations. In many cases, hepatitis B does not present symptoms, but warning signs can include jaundice, fever, muscle and joint aches, and dark urine. Treatments exist to control the virus and reduce liver damage, and in severe cases a liver transplant may be required. The global vaccination effort has seen more than 1.4 billion doses administered to date.