Another New Hampshire man receives experimental pig kidney as U.S. trials prepare to begin
Massachusetts General Hospital reports patient is faring well as FDA clears gene‑edited pig kidney study and other developers prepare similar trials
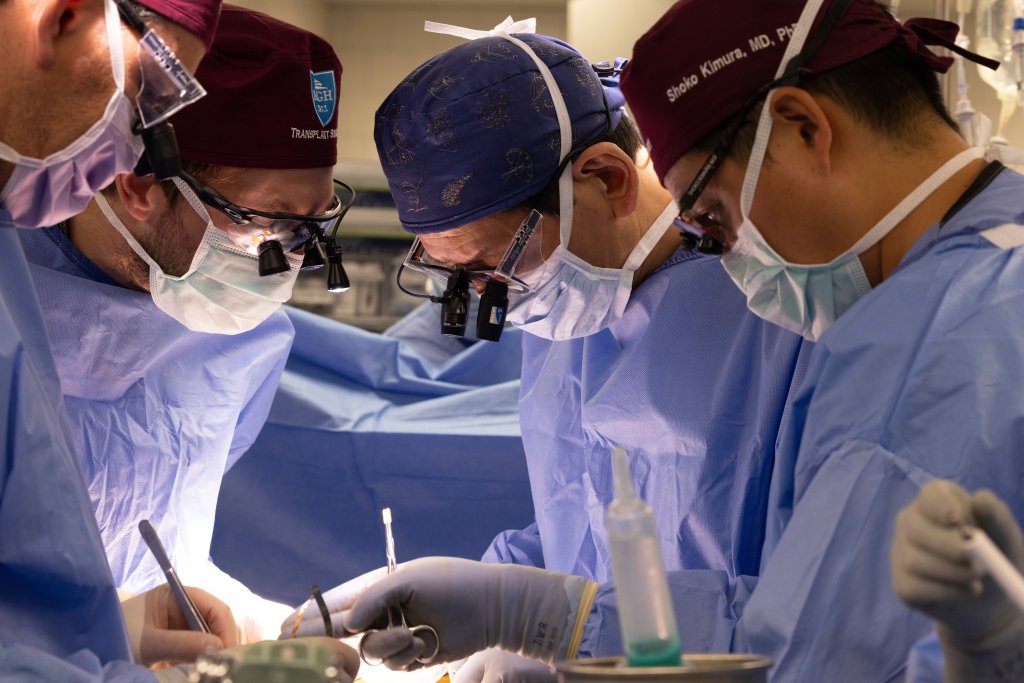
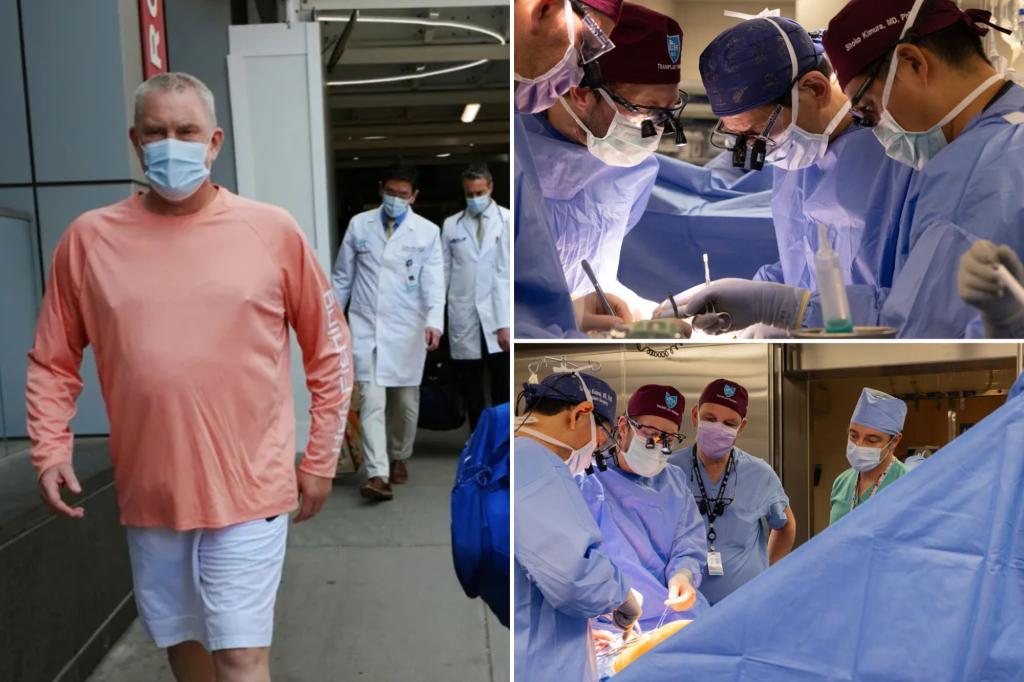
A 54-year-old man from New Hampshire who said he was motivated by science received an experimental, genetically edited pig kidney at Massachusetts General Hospital on June 14 and is recovering well, hospital officials said Monday. The procedure comes as the Food and Drug Administration has cleared a company to begin a formal clinical trial of pig kidney transplants, a step toward testing whether animal organs can help reduce the chronic shortage of human organs for transplantation.
Bill Stewart, an athletic trainer from Dover, New Hampshire, told The Associated Press he applied to the Mass General program because he wanted to "contribute to the science of it." Stewart had high blood pressure that led to kidney failure, spent two years on dialysis and faced long waits for a human kidney; physicians have said it can take up to seven years for people with his blood type to receive a matching organ from a deceased donor.
The Mass General team noted the operation is part of a wave of carefully controlled attempts to see whether gene‑edited pig organs can function in human bodies long enough to provide meaningful benefit. Another New Hampshire recipient, Tim Andrews, has remained off dialysis for seven months after receiving a pig kidney at Mass General, a record duration for such a transplant, the hospital said. Before Andrews, the longest documented survival of a gene‑edited pig kidney in a human recipient was 130 days, in a widely reported case involving a patient in Alabama.
The FDA recently approved eGenesis, a U.S. developer of gene‑edited pig organs, to begin a regulated clinical trial that will provide pig kidney transplants to 30 people aged 50 or older who are on dialysis and on the transplant list. United Therapeutics, another developer in the field, is preparing to enroll patients in a similar, FDA‑approved study.
"Right now we have a bottleneck," said Dr. Leonardo V. Riella, a kidney specialist at Massachusetts General who will help lead the eGenesis trial. More than 100,000 people are on the U.S. transplant waiting list, most of them seeking kidneys, and thousands die while waiting, Riella said. Gene editing of pigs aims to alter animal organs so they are less likely to be immediately attacked by the human immune system and more compatible with human physiology.

Early xenotransplant experiments, including two heart and two kidney transplants, were performed in very ill patients and were short‑lived. Chinese researchers have also reported a kidney xenotransplant but released little clinical detail. The Alabama case, in which a pig kidney functioned for 130 days before rejection led to its removal and the patient's return to dialysis, prompted researchers to shift toward enrolling less critically ill candidates to obtain clearer safety and efficacy data.
Stewart said he consulted Andrews before the operation and was aware of the experimental nature of the procedure. After the transplant, he reported feeling less burdened by treatments and has returned to some desk responsibilities at work. Mass General doctors said they adjusted Stewart's anti‑rejection medication after early concerns and have made similar adjustments for Andrews.
"A year, hopefully longer than that — that's already a huge advantage," Riella said, noting that even temporary function of a pig kidney could spare patients the time, expense and health toll of dialysis while they wait for a human organ.
The upcoming eGenesis trial will enroll adults older than 50 who meet specific criteria and are already on dialysis and the transplant waiting list, according to hospital and company summaries of the planned study. Investigators have emphasized careful monitoring for immune rejection, infection and other complications, and regulatory reviewers have required robust safety protocols before allowing enrollment to begin.
Researchers say xenotransplantation raises clinical and ethical issues, including long‑term monitoring for infections that could potentially cross species and equitable selection of trial participants. Proponents argue the technology could dramatically increase the supply of organs, potentially saving thousands of lives if safety and efficacy are demonstrated in rigorous trials.
As the field moves from isolated, compassionate‑use operations toward formal clinical studies, scientists and clinicians say caution and close oversight will be essential. The results of the eGenesis trial and similar studies by other companies will be watched closely by regulators, patients and transplant centers seeking alternatives to a persistent organ shortage.